Jordan: Ancient Metallurgy
Content created: 2001-10-31, revised 2014-04-15
File last modified:
Ancient Metallurgy
An Overview for College Students
Related Pages:
Ancient Cloth,
Basic Stone Tools
Metallurgy is the process of working metal into artifacts (tools and toys). Although small amounts of metals are found in relatively pure form, most must be extracted from more complex ores by removing the "impurities" (non-metal or other metal) from the combination ore.
It is possible, of course, to pound on metal ores and chip off pieces, and a few very early "chipped stone" tools were in fact made of chipped ore. It is also possible to reshape raw ores slightly by pounding —depending upon the hardness of the alloy— and the result can sometimes be used as a tool. Metal ores processed in these ways have never been significant in human history, however. (For example, compared with chipped obsidian, chipped iron ore makes a far less usable tool.) Instead, usable metal tools involve heating and/or hammering the metal to work it into something usable.
Page Outline:
Return to top.
Materials: Copper

Copper Nugget, 2,492 kilos (5,495 pounds), found during 1936 mining operations in the Wrangell Mountains, about 300 miles east of Anchorage. It is extraordinarily rare to find a nugget of copper this large or this pure.
Museum of the North, Fairbanks
Copper (abbreviated Cu) is a relatively soft, pinkish-reddish metalic element. It is technically a "transitional element" rather than a metal, although it is conveniently regarded as a metal in historical contexts. One of the most common carbonates of copper is malachite, which was used in antiquity as a gem stone.
Copper is generally corosion-resistant. But when it is exposed to air, the exposed surface oxidizes, changing the red-pink color, or it interacts with ambient sulphur to produce a thin "patina" of blue-green copper-sulphate.
Copper is rarely found completely unmixed with other material. (Click me.) But it nevertheless sometimes does occur in nearly pure nuggets. Like other metals, it turns up occasionally in Paleolithic contexts, when people tried to work it by stone knapping techniques. However, it was not worked as a metal until perhaps 6000-5000 BC. The earliest use was for beads and other ornaments, since copper by itself is too soft for the production of much in the way of useful tools.

As it comes from the mine, copper, like other mined metals, is quite impure.
Private Collection
Despite this limitation, copper came to be more and more widely used, and archaeologists, especially those working in the Near East, sometimes speak of a Copper Age or "Chalcolithic" (kal-ko-LITH-ic) period from about 5000 to about 3000 BC in that region, or from the end of the Neolithic to the beginning of the Bronze Age. (Others prefer the term "Eneolithic" for this period.) (Obviously not all parts of the world were on the same schedule, or even used copper enough for a term like "Chalcolithic Period" to make any sense more generally.)
As a sign of how important trade in copper became, the Latin word Cuprum, "copper," was apparently originally the name for the island of Cyprus, known for its copper mining, somewhat as the English term "china" derives from the name of the country which provided the world with particularly fine ceramics.

Cross section of a piece of copper ore, showing high admixture of other material.
Private Collection
As noted, copper ore, although found in many regions, is rarely pure. (And large nuggets like the one shown above are very rare.) Therefore it is of little use until it can be removed from its entanglement in other material. Copper melts at about 1,083°C (1,981°F). As ancient pottery workers experimented with the effects of higher temperatures on ceramics they began also to develop methods that could produce a high enough temperature that copper could be melted sufficiently to be separated from the other materials with which it was found. (See below, smelting.)
It was probably early noted that in some cases small admixtures of certain other materials strengthened copper products or made the metal easier to work, and various alloys are found, some of which may thereafter have been deliberate. Indeed, the distinction between impure copper artifacts and early bronze ones is not always immediately obvious.
Return to top.
Materials: Bronze

Because of its high copper content, bronze, normally reddish in color, gradually develops a blue-green patina as the surface interacts with oxygen and other elements in the air. The unstable patina can be often be rubbed or eroded off and may therefore be uneven, as shown in this XXth-Century Chinese Bronze Figure
Private Collection
By far the preferred copper alloy in antiquity was bronze. Bronze is a combination of copper and tin, usually roughly eight or nine parts copper to one part tin. In some cases additional elements were added as well. As noted, copper itself is quite a soft metal, and only marginally useful for tools. So, for that matter is tin. But the introduction of tin into copper produces hardness greater than that of either metal alone, as well as making casting easier.
Increasing the amount of tin in the alloy much above 10% produces greater brittleness, and tools made that way easily break. However alloys with more tin —potin (up to 20% tin) and speculum (more than 30% tin)— were used in some parts of Europe for early coins, since brittleness was not a significant problem in the case of coins. (Many variations occur, since the purity of the raw materials was difficult to control.)
Copper is found in many places in the world, although not universally. Tin, in contrast, is actually rather rare. Thus the production of bronze depends upon felicitous location and/or successful trade, and it was always a luxury product in the ancient world, even when "useful" products were made from it. Control of copper and tin mines, and of associated trade routes, therefore became important to the wealth and prestige of many peoples, and, when bronze weapons became widespread, important to their security as well.

Bronze is favored for public statuary, but is not indestructable. The oxygenated copper patina not only turns green, but can be carried by rainwater runoff and even stain a stone base.
Antimonial Bronze
Simple bronze, made only of tin and copper, does not normally produce a sharp edge (although it can be sharpened by beating). The introduction of antimony in addition to the tin and copper produces a harder bronze, better able to hold a cutting edge and less likely to be bent in use.
Arsenical Bronze
Like antimony, arsenic added to the tin and copper (up to as much as 3% of the whole) produces a harder final product. Arsenic fairly routinely occurs as an impurity in early bronze anyway, and small amounts of it were probably not intentional or particularly noticeable in the final product. By the time the proportion of arsenic in bronze reaches two or three percent, however, the effects are quite noticeable and presumably intentional. It is to these products that the term "arsenical bronze" is usually applied.
Lead Bronze
Mixing lead into the copper-tin alloy produces "lead bronze," which may contain as much as 10% lead. The lead in the alloy does not become part of its crystalline structure, increasing the fluidity of compound when it is in its molten state. This facilitates casting, particularly the casting of finely detailed artistic objects, such as those produced as elite status symbols in early China. However lead bronze is softer than normal bronze, and therefore less able to hold a cutting edge, making it less appropriate for many types of tools.
The Bronze Age
The term "Bronze Age" refers to those periods around the world in which bronze was in general use. The specific dates of course vary from region to region, and vary also with the rigidity with which one defines "general use." The Bronze Age in any given place is considered to have come to an end when the generalization of iron brought on the beginning of the Iron Age, an equally problematic term.
Brass
Brass is an alloy of copper with zinc, and is usually made up of anywhere from ten to forty percent zinc. Small amounts of other ores produce special-purpose brass. (Tin and aluminum increase resistance to corrosion, for example.) Zinc ore (called calamine) is difficult to mix with the copper ore, however, and brass appears later in the archaeological record as well as being far less common than bronze.
Return to top.
Materials: Iron & Steel
Iron is one of the commonest (and cheapest) metallic elements on the planet. In fact, by weight it makes up about 5% of the planet's crust, where it is found as a range of slightly impure "iron ores": hematite, limonite, magnetite, etc.
It was not until about 1200 BC that iron became general for the production of tools because the temperatures needed to process the ore exceeded what most ancient kilns were able to reach. Pure iron has a melting point of 1,535°C. The limit of an ancient furnace was about 1,150°C. (Recall, by contrast, that copper has a melting point of 1,083°C. Ancient pottery kilns sometimes also reached temperatures in this range, but it was rare to get far above it.)
It was eventually discovered that the introduction of three to four percent carbon to the mixture could sometimes lower the melting temperature (ultimately to as low as about 1,150°, hence just at the furnace limit). Unfortunately, carbon also tended to contribute to the brittleness of the resultant products. Therefore the controlled introduction of carbon into iron ore remains a critical aspect of iron and steel production. (Carbon is not used in the production of bronze.)
Carbon was not the only technological innovation involved, however. Furnace structure and fuel were important in reaching the necessary temperatures. Hardwoods, such as those of the central African area, burn hotter than softer woods. (This is probably the reason for the especially widespread mastery of iron in some areas of Africa.) Further, the use of charcoal in place of wood allowed a yet hotter fire. So did the eventual discovery of coal as a fuel. Similarly, the use of bellows to force air into the kiln produced more rapid burning and faster release of heat by increasing the oxygen available to the fire.

XIXth-century iron cooking pot with iron stand, from Wales. Virtually identical items were common throughout northern Europe and North America.
Museum of Welsh Life, Cardiff
Carbon-free iron heated to the maximum of ancient furnaces, while still not actually reaching its melting point, could be pounded (forged) to purify it and shape it, even without any admixture of carbon. Iron worked in this way and containing only negligible carbon is called "wrought iron," and its production is necessarily quite time-consuming. On the other hand, iron with high carbon content and as a result with a lower melting point could be melted and molded and is referred to as "cast iron." In Europe successful iron casting dates only to about the 1300s AD. However, in China iron casting dates to the 500s BC, when cast iron began to be used for the production of agricultural implements.
Even once the technology was known, manufacturing iron was not easy and could easily fail if the ore was not sufficiently porous or if there was too much oxygen available or if the lumps of charcoal used to introduce carbon were too large. It is important to remember that throughout history more groups knew about iron, and valued it, than could produce or work it. (Click me.)
The term "Iron Age" is given to those periods around the world in which iron came into general use. The specific dates of course vary from region to region, and the rigidity with which one defines "general use." One date sometimes given for the earliest iron production is about 2000 BC (for Turkey). Iron appeared in Africa by 600 BC, probably from Southwest Asia via Egypt, Nubia, and the Sahel corridor running south of the Sahara, and substantial iron working began in what is today Nigeria by the 300s. In most parts of the world, the use of iron largely displaced the prior use of bronze, and hence the "Iron Age" succeeded the "Bronze Age." Except in the northeast, there was no Bronze Age in Africa, where iron directly displaced the use of stone tools.
Steel is an alloy of carbon and iron (the metallic element, not the finished product). It contains less carbon (0.2 to 1.5%) than cast iron, but more than wrought iron. High-carbon steel is harder and more brittle, while lower carbon content makes the product softer and easier to work.
Usually traces of such other metal oars as chromium, nickel, copper, tungsten, etc. are also added to produce kinds of steel with slightly different characteristics.
The production of steel requires the removal of more of the impurities in iron ore than iron production does, often through the application of greater heat than ancient furnaces could produce.
It is easy to think of steel as iron ore to which carbon is added, but in actual production it was often cast iron from which carbon was removed, a process called "decarburizing," and in China several processes for accomplishing this were used beginning in the Hàn dynasty 汉 (206 BC - AD 220).
In general, steel is an improvement over iron in being less brittle, but its characteristics vary by the amount of carbon in the alloy. The introduction of other metallic ores allows the production of special purpose steels, such as stainless steel, made with chromium.
An important technique in modern and late historic steel production is "quenching," that is, heating the metal and then rapidly lowering its temperature again by plunging it into water. (See below.) The result is a dramatic increase in the strength of the metal, strength which can be increased yet further by repeating the process. The earliest quench-hardened steel that we know about dates from about 1200 BC or so. (Homer refers to the process.) But steel was too difficult to produce dependably to come into wide use at that point.
Obviously there is a fine line between iron and steel, and some metallic products are difficult to classify as quite one or quite the other. Techniques for raising furnace temperatures, controlling carbon content, and quenching after raising the metal to just the right temperature were central to the production of iron-ore based tools that were actually superior to bronze ones rather than merely cheaper.
Return to top.
Materials: Lead

This lidded baptismal font, dating from 1170, was cast in soft lead. Lead was used for baptismal fonts in Britain starting in the 300s.
Abbey Church of Saint Peter and Saint Paul, Dorchester, England
Lead is rarely found by itself, but rather is usually a by-product of the processing of other ores. It is also the final stage of radioactive decay of some unstable metals, such as uranium or radium.
Dull gray in color, lead is quite soft. For example, in an account of her father's hunting in the mid-1800s, Waheenee, a Hidatsa woman, remarks that, "For shot he used slugs, bits of lead which he cut from a bar, and chewed to make round like bullets. Powder and shot were hard to get in those days." (Wahenee 1927:18)
Lead also has a low melting point, which makes it a ready alloy with other metals, as well as a good metal for casting artifacts at comparatively low temperatures. In addition, lead is quite heavy, so that it is well used in weights. Thus archaeologically we find lead artifacts from quite early times.

The use of lead in ceramic glazes can be dangerous, especially when the firing temperature is low or the glaze extends to the mouth of a vessel. Early XXth-century Mexican pottery, like this piece, acquired an unsavory reputation among tourists and Mexicans alike despite its attractiveness.
Various metals, such as tin, were (and are) sometimes combined with silica in ceramic glazes, to which they lend a hard, shiny surface, the color of which can be controlled by the combination of materials. (The most famous example is faience [faïence].) However most metals have a melting point above the capacity of the ancient kilns (or in some cases simple dung fires) used in ceramic manufacture. An admixture of lead could create alloys with a melting point within the range of relatively low-temperature ceramic production. When higher temperatures were available, lead still often increased the flexibility of the glaze as well as its lustre. For this reason, lead has long been associated with the production of spectacular pottery glazes and porcelain ware.
Lead suspended in paint can also help to produce a hard, usually shiny, long-lasting surface.
Lead is a toxin, which builds up gradually in the body until it hits a threshold amount and begins to produce symptoms. Unfortunately, the effect is gradual enough that for many centuries what we now know to be lead poisoning was not associated specifically with lead. Gradual lead poisoning can result from water carried through lead pipes such as those sometimes used in ancient Rome, for example, or from wine served from lead vessels, although the toxicity is apparently considerably less (unto negligible) in most alloyed forms.
Because lead has been used as an ingredient in ceramic glazes, it has proven a source of potential poisoning to users of ceramics fired at low temperatures. Some foods, such as orange juice, interact with lead glazes to draw the lead into food especially quickly. For this reason lead is now normally excluded from ceramic glazes by most potters, and those who do make use of it confine it to external decoration of objects not intended to contain foods.
Return to top.
Materials: Pewter
Pewter was traditionally an alloy of tin with other soft, workable metals with low melting temperatures, such as copper, antimony, or sometimes lead. It is the softness of the metals in the alloy that accounts for the ease with which pewter utensils can be artistically worked, but the same softness means they are easily dented.
Many of us associate lead with pewter, and most dictionaries describe pewter as an alloy of tin and lead. It appears that in antiquity that was largely the case. However, modern pewter producers take pains to assure buyers that their pewter does not contain lead. In fact, some pewter has always been lead-free. A visitor to this web site provided the following additional information:
Fine pewters of the 16th, 17th, and 18th centuries were basically alloys of workable, low melting metals, with tin as the base metal and additions of copper, antimony, and sometimes bismuth.
It was only in the "cheaper" pewters that lead was present, for example "lay metal" (20% lead) and "black metal" (50% lead). Such "cheaper" pewters presented a toxicity problem when used as food/drink containers since lead could leach out.
These pewters were superseded in the mid 1700's by "Britannia Metal", an alloy of tin (85%), Antimony (10%), zinc (3%), copper (1%), and sometimes bismuth.
Return to top.
Materials: Gold
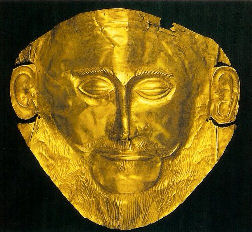
This famous gold mask from Mycenae is an example of the use of thin gold sheets designed to get maximum surface glitter with a minimum of material.
Mycenae, Greece
Gold occurs alone or in admixture with other ores. It is soft and easily worked, and does not react chemically in most circumstances. It is largely immune from tarnishing or other surface deterioration.
Thus from earliest times gold has been considered to be "incorruptible," and has been treasured as much for that (and the associated symbolism) as for its comparative rarity and its surface glitter.
However gold is quite soft, and gold objects are accordingly rather weak. Therefore it is usually either alloyed with other metals (such as copper) or used as a surface covering on objects made of sturdier (and cheaper) material.

Gold processed into flat flakes and placed between sheets of shiny, non-absorbant paper to be pounded flatter.
Central Myanmar
Gold hammered into thin sheets can be pressed or pounded over or into a mold to produce a bas-relief design, a process known as repoussé. (Less commonly repoussé is produced by placing the gold sheet on a soft surface and striking it with a hammer bearing the desired design.)

An artisan repeatedly hammers a gold sheet, protected by non-absorbant paper and fixed in a frame, until it reaches an almost powder-like thinness for use as gold leaf.
Central Myanmar
The high cost and comparative softness of gold means that many ancient objects, such as crowns, bracelets, and burial masks, were made this way. Unfortunately, they were quite fragile and foil-like, and in archaeological contexts one often finds that they have been crushed or crumpled.
Gold can be worked into even thinner sheets and applied as foil — gold leaf — to produce a thin metalic veneer on objects made of wood or other material, and indeed that is perhaps its most common form archaeologically preserved. This allows the possibility of using gold as a surface on internal and external architectural details, from palace door frames to roofs of temples, as well as smaller objects ranging from cosmetic jars to book covers.

"Gold leaf" is used as a surface over other materials in furniture and in architectural decoration.
Lintel Decoration, Rundāle Palace, Pilsrundāle, Latvia
As early as the 2000s BC, "depletion gilding" seems to have been done in Mesopotamia. In this technique an alloy of gold and silver was affixed as a thin foil over a copper base.
Next the object was coated with a material (such as salt) that would corrode away the silver, which could be gradually washed off as a black powder. Finally the gold could be gently buffed. This technique was perhaps not appropriate for very fine work like the gold-leaf examples shown here, but the surface was resilient and the object appeared to be (and perhaps could be passed off as) solid gold. (Depletion gilding is difficult to see archaeologically, since what remains is the gold and the base, the silver having vanished in the course of production.)

Wooden book cover for the Diamond Stura, coated with gold leaf. Tibet, XVIth century. Because gold can be hammered so thin, gold leaf makes possible gold-covered objects in a way that is unlike any other metal.
Norton Simon Museum, Pasadena
In modern times, the process of electroplating allows the application of a layer of gold as thin as a single atom deep on appropriately receptive surfaces. Thus a very thin layer of gold is now used to improve the conductivity of computer connections, as a plating on even relatively cheap jewelry, or as a thin overlay on sashimi eaten as a status symbol by pretentious diners in Japan.
Lumps of gold, in contrast, are extremely dense and heavy. Many Chinese believe that eating a lump of gold was an aristocratic method for committing suicide in imperial times, since the heavy lump would overwhelm the digestive track and produce internal bleeding and death.
An alloy of gold and silver (which can occur naturally or be deliberately created) is called electrum and was sometimes regarded as more valuable than pure gold. Gold can also be alloyed with copper in various proportions to produce tumbaga, which was much used in ancient Peru. Since the color of tumbaga varied with the proportion of copper included (normally less than 50%), Peruvian goldworking could make use of the color contrasts to produce designs in the goldwork.
Return to top.
Materials: Silver
Unlike gold, silver rarely occurs in a pure form in a sufficient quantity to be useful. Usually it has been procured by smelting other ores, of which it can be thought of as almost a by-product. In antiquity a common ore used to produce silver was galena, or lead sulfide, although it appears that lead carbonate (cerussite) was also used.

Silverworking in Central Myanmar.
After the bowl is shaped, the surface designs are crudely pounded in from the inside, after which they are finished by gentle tapping on the outside.
As was the case with gold, silver was always rare enough that almost everything made from it was a decorative or prestige item rather than a tool as such.
Silver, like lead, is a very soft metal, easily damaged when used by itself, and it was therefore often alloyed with other metals to increase its hardness. The admixture of a small amount of copper was usual for this purpose. Most alloys of silver remain quite soft, however, and are comparatively easily worked into art objects. Because of its high value combined with its softness, silver is often used in thin plates, suitable for jewelry, picture frames, decorative vessels, and the like, or it is attached to a stronger backing material. Silver cannot be pounded into as thin a layer as gold, and therefore it cannot easily be pressed or dusted over a surface such as carved wood or plaster without losing the design of the underlying carving.
Return to top.
Methods
 Above:
Above: Ingots are simply metal blocks of any convenient shape, raw materials for artisans.
Below: In China distinctively shaped ingots came to be used as money. The XIXth-century example below represents 50 official taels, about 1.9 kg, of a standard-grade silver alloy.
British Museum

Mining & Ingots
It is extremely rare for any metal to be found in a pure state, and therefore mining pulls up a good deal of additional material as a by-product. The first step in metalworking always involves separating the desired metal from the raw ore in which it is naturally found, and then using the metal (sometimes alloyed with other metals) to manufacture something.
Virtually all metalworking involves heating the metal to soften or melt it, and in antiquity the temperatures required often represented the very limits of available heating technology. Not all metals could be completely melted because ancient furnaces simply couldn't get hot enough.
Logically, removing the impurities from metal (smelting) and actually making it into something do not have to be done at the same time, and it has always been common to process newly extracted metal into blob-shaped or bar-shaped ingots, convenient for storage or shipping.
Later, when it was time to manufacture something, the material in the ingot needed only to be heated and it was immediately ready for use.
 Above:
Above: First-century Roman ingots of (left to right) copper, tin, and lead. (
Musée d'Arles)
Below: Glass ingots from a shipwreck of the 14th century BC. (
Underwater Archaeology Museum, Bodrum, Turkey)

Obviously, this required heating the metal twice: once to purify it and make the ingots, and later to melt the ingots for reworking. But there were advantages. Ingot production could be carried out by cheap labor near a mining site, and the considerable mining waste (or slag) could accumulate in debris mounds there, typically far from most centers of human habitation. Actual craft production could be located closer to the intended market, in more heavily inhabited places, far from the ore sources. And, perhaps even more importantly, only the ingots needed to be moved; there was no the need to transport waste material.
(Glass was also stored in ingots and then melted and cast in molds as needed, separating the sometimes secret process of glass creation from that of glass working. The picture here shows glass ingots tinted with traces of copper, cobalt, and manganese.)
Return to top.
Smelting
Smelting is the basic process by which one produces workable metal from metal ores. The minerals in copper ores are reduced to copper through mixing carbon with the ore and heating the combination to about 1,100°C. (This can be done directly with copper oxide ores. Copper sulphide ores are heated in contact with air first.) At this temperature the metal, now liquid, flows to the bottom of the furnace, and the remaining matter (slag) floats to the top, whence it is removed. (Slag usually includes large amounts of silicon and related material and produces waste heaps of glass-like or cinder-like material.) Although this sounds straightforward, in antiquity, and especially before the invention of a bellows, it was difficult to attain the necessary temperature, and the extraction of copper from slag was in fact a difficult, messy, and extremely labor-intensive project. (Click for pictures.)

This "tailing" or bit of cinder-like debris from a modern copper refinery contains a wide range of materials.
Private Collection
Smelting produces a blob of metal (called bloom) prepared for the next step. In the case of copper, that step is often casting. (See below.)
Iron has a higher melting point than copper. But below its melting point iron can still become spongy and amenable to treatment by hot hammering (forging), which helps to extract some of the remaining impurities. (See below.)
Cold Hammering
Softer metals, including copper and bronze, can be shaped through cold hammering, especially after they have been smelted to remove impurities. Because hammering is harmful to the crystalline structure, hammered metal becomes more brittle. The crystalline structure can be "reset" by annealing, a process of successive heating and slow cooling, described below.
Forging (Hot Hammering)
Forging iron, which is much harder than copper, requires that the bloom be reheated until it is red. It is then hammered on an anvil, a process that physically drives out the various impurities (usually silica) that remain from the smelting. Repeated heating and hammering produces ever purer (and stronger) iron. At the same time, since the iron is slightly malleable when it is red hot, the smith is able to shape the iron into desired forms.
Hot hammering does not work with bronze, which becomes too fragile at high temperatures.
Return to top.
Annealing
 Above:
Above: Clay mold for casting a sword blade, from Bronze-Age Britain.(
Ashmolean Museum, Oxford)
Below: Iron mold for casting two bronze sickles, from Central China about 300 BC. (
Chinese National Museum, Beijing)

Annealing is the process of reheating cast or hammered metal slowly until it is red hot. This restores its crystalline structure and is necessary after metal has been repeatedly hammered. Hammering shapes the metal and drives out impurities, but also weakens its crystalline structure, making the metal hard but brittle and easily cracked. In the case of tools where sharp edges are important (such as knives), it is sometimes preferable to leave them unannealed after the final hammering, since the hardness (and hence the ability to hold a sharp edge) may be more important than the potential brittleness (and hence the tendency to break).
Quenching
Quenching, as mentioned above, refers to heating iron (alloyed with carbon) to a high temperature —at least the 725°C threshold where it turns red— and then rapidly cooling it by plunging it into water. This process has no useful effect on pure iron or on bronze, but for iron with some carbon admixture, it produces a great increase in hardness, and it became a routine part of the production of such objects as steel swords.
Casting
Fully molten metal can be cast, that is, poured into a mold, so long as the mold has the structural integrity to withstand the heat. Since iron did not fully melt at the temperatures ancient metalworkers were able to produce, iron was normally worked by other methods (outside of China), but lead, copper, and bronze were usually cast, and molds have been found from metalworking cultures around the world. (The difference in melting temperatures made iron a possible material from which to make a mold from which to produce tools made of other metals. Although such a mold would be difficult to produce, it could be used for a long time.)
A specialized method of metal casting, used in the production of particularly complicated objects, is called the lost wax technique.
Return to top.
Two interactive review quizzes are available to doublecheck your understanding of topics on this page:
Quiz 1
Quiz 2.
Sources
This page is based on a wide range of sources, many of them long forgotten, and on advice from several friends and colleagues. With the exception of the Mycenaean mask, all pictures were taken by me. Among the most useful books have been:
- ANONYMOUS
- 1987 The manufacture of metal in the ancient Near East. Washington, D.C.: Smithsonian Institution.
- CHESHIRE, Gerard
- 2001 Chemical elements. London: HarperCollins.
- WAHENEE
- (1927) 1981 Wahenee: an Indian girl's story told by herself to Gilbert L. Wilson. Lincoln: U. of Nebraska Press.
- WHITEHOUSE, Ruth D.
- 1983 The Facts on File dictionary of archaeology. Revised edition. New York: Facts on File.
- WILLIAMS, Brenda
- 2006 Ancient Britain. Norwich: Jarrold Publishing.
Return to top.
There have been
visits to this page since 160901.
Background Design: Chīyóu 蚩尤, Mythical Creator of Chinese Metallurgy & Weaponry
(based on folk-art paper cutout)



 Because of its high copper content, bronze, normally reddish in color, gradually develops a blue-green patina as the surface interacts with oxygen and other elements in the air. The unstable patina can be often be rubbed or eroded off and may therefore be uneven, as shown in this XXth-Century Chinese Bronze Figure
Because of its high copper content, bronze, normally reddish in color, gradually develops a blue-green patina as the surface interacts with oxygen and other elements in the air. The unstable patina can be often be rubbed or eroded off and may therefore be uneven, as shown in this XXth-Century Chinese Bronze Figure
 XIXth-century iron cooking pot with iron stand, from Wales. Virtually identical items were common throughout northern Europe and North America.
XIXth-century iron cooking pot with iron stand, from Wales. Virtually identical items were common throughout northern Europe and North America. 












 Above: Clay mold for casting a sword blade, from Bronze-Age Britain.(Ashmolean Museum, Oxford)
Above: Clay mold for casting a sword blade, from Bronze-Age Britain.(Ashmolean Museum, Oxford)