Rather than create a new vocabulary to refer to concepts which have long been studied in other disciplines, electrophysiology borrows terms from physics and chemistry. This can result in some confusion for the cognitive science student unfamiliar with those subjects if the terms are not properly defined.
A potential (or voltage) is the amount of work necessary to bring two charges of some initial separation and magnitude together. Like charges repel each other, whereas opposite charges attract one another. The force between two charges is inversely proportional to the distance between them. Often, the potentials of many ions are spatially summed. It is assumed that the distance between the charged regions is constant and the focus is on the variable magnitudes of total charge in each region. This assumption allows extension of the concept of potential to the amount of charge difference between two regions, such as the intracellular and extracellular environments.
A current is the flow of charge from one point to another. Currents conduct graded potentials and carry the action potential between Nodes of Ranvier.
Conductance measures the ability of a conductor to pass along an electrical charge without diminishing the magnitude of that charge by losing potential energy. There are two types of conduction: decremental conduction, in which the signal is quite fast but the voltage is not regenerated and thus decreases over time, and non-decremental conduction, in which the signal is very slow but is constantly regenerated so as not to lose magnitude. Myelin sheaths on axons improve their conductance by insulating the axon and improving action potential propagation.
The transmission of signals within a single neuron is essentially electrical. Unlike the electricity which runs through wires and involves the flow of electrons, in the cell it is the flow of charged particles, or ions, that is the basis of the electrical potentials. The most common ions in the cellular environment are Na+, K+, Cl-, and Ca++. These ions are unequally distributed across the neuron’s membrane. In particular, Na+, Cl-, and Ca++ are found with higher concentration outside of the cell, while K+ and various negatively charged ions called anions, are at a higher concentration inside the cell (when the cell is at rest).
In order for a change in the basic electrical state of the cell to be recognized as such, there must be some baseline from which deviations are measured. This baseline state is called the resting membrane potential of the cell. All neurons have resting potentials, as do many other types of cells in the body. The resting potential in a typical neuron is approximately -65 mV (this means that the inside is more negative than the outside).
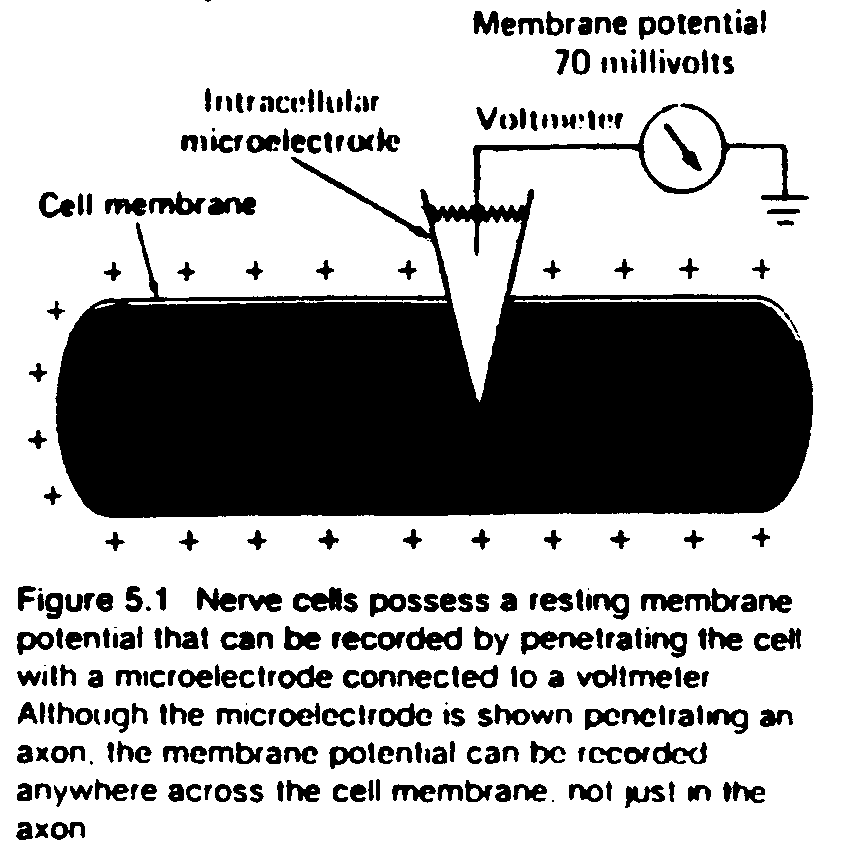
The resting potential arises from two forces: osmotic pressure and electrostatic force. These forces are a consequence of the different concentrations of ions inside and outside the cell . The tendency of ions to move from areas of greater concentration to areas of lesser concentration is called osmotic pressure. Ions also tend to flow to areas of opposite electric charge. Negative ions are attracted to more positively charged areas, and positive ions are attracted to more negatively charged areas; the force this tendency produces is called electrostatic force.
Sometimes these two forces work against each other. Potassium, for example, is present in greater concentration inside the cell, which is negatively charged in comparison with the extracellular space. Thus osmotic pressure would lead it to exit the cell, but electrostatic force would lead it to enter the cell. The cellular membrane is most permeable to potassium, but it is also slightly permeable to sodium. When the forces acting on the ions which can cross the membrane (mostly potassium) reach a balance, a dynamic equilibrium is formed. This occurs when the number of ions flowing out is exactly equal to the number of ions flowing in; it is the resting potential.
The Nernst equation allows one to calculate the membrane potential based on a single ion. If one calculates the Nernst equilibrium potential for potassium in the cell, the result is about -70 mV, which is close to the resting potential of -65 mV. The Goldmann equation takes into account all the ions present, rather than just one, and thus is more precise in calculating the membrane potential of a neuron.
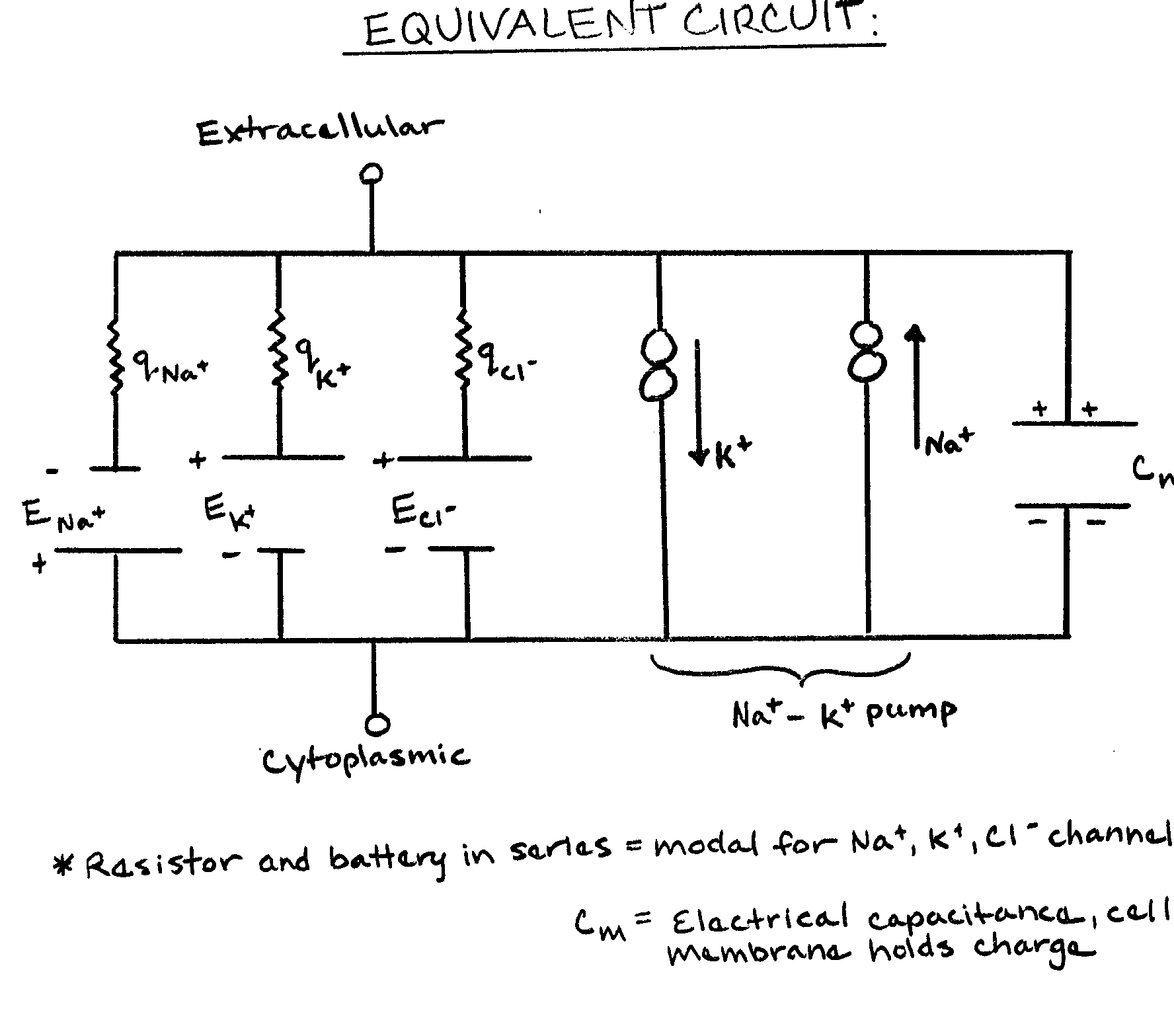
As mentioned before, the membrane is most permeable to potassium ions. This is because proteins embedded in the membrane, called passive ion channels, allow potassium ions, but not sodium ions, to cross the membrane. However, there is a small but significant leakage of sodium ions across the membrane into the cell. If this were allowed to continue indefinitely, the membrane potential would eventually disappear. To combat this leakage, sodium-potassium (Na+-K+) pumps exist. These are specialized transport mechanisms that exchange three sodium ions (out of the cell) for two potassium ions (into the cell). This restores the appropriate concentrations of ions inside and outside the cell.
Na+-K+ pumps use 30-40% of the energy of a cell. The energy is obtained from the breakdown of adenosine tri-phosphate (ATP). Due to the electrical nature of the membrane potential, one can model the neuron as an equivalent circuit. This circuit must take into account the various ions present, the sodium-potassium pump, and the membrane capacitance.
Classical electrophysiology made a number of assumptions about the nature of neurons and the signals which they utilize. One basic assumption was that cells were essentially alike as far as electrophysiology goes. The number of ion channels recognized was small, and they were assumed to be uniformly distributed along the membrane. Likewise, very few neurotransmitters were recognized. The action potential was considered the important signal; graded potentials were merely discrete negative or positive signals which produced the action potential.
These assumptions implied certain conclusions which somewhat oversimplified the brain. Neurons were considered quite simple and "dumb" units which performed rote calculations and spit out the only important product, the action potential. In this view each neuron had only two things to contribute: yes or no. The enormous complexity of the brain was considered a result of how the vast numbers of "dumb" neurons it contained were connected; sheer numbers, rather than individual complexity of neurons was considered the key. This hardly does justice to the intricacy of the single neuron. It is more than just a data register, and more modern views take this in to account.
Researchers now know that there are many different ion channels, both ligand-gated and voltage-gated. For example, twelve different types of potassium channels alone have been identified. These channels differ in aspects such as time course (the amount of time required for an ion to pass through), sensitivity to membrane voltage, and sensitivity to neurotransmitters. The various channels are distributed in a specific pattern rather than uniformly or randomly. Higher threshold calcium channels, for example, are found more in the dendrites, whereas their lower threshold counterparts are found more in the soma. The density of channels in a particular location is not fixed, but rather changes as needed. Ion channels are also replaced constantly without jeopardizing the cell's stability; this is called up and down regulation.
Action and graded potentials have not escaped scrutiny, either. Graded potentials are now recognized as carrying information, and some researchers think that they may even be more important than action potentials. With this in mind, graded potentials will be the next topic under consideration.
When a presynaptic cell releases a neurotransmitter which binds to receptors on the postsynaptic cell, a graded potential results. The mechanisms by which graded potentials are produced are discussed in Module 7 (Neurotransmitters); for now we are concerned with what happens after the graded potential is created.
Graded potentials can be classified as either excitatory or inhibitory. Excitatory post-synaptic potentials (EPSPs) cause a depolarization, making the membrane potential less negative. Inhibitory post-synaptic potentials (IPSPs) cause a hyperpolarization, making the membrane potential more negative. The neuron algebraically combines these inputs to produce an action potential.
Graded potentials move relatively quickly, as they are driven by passive ion currents. If one artificially stimulates a cell, the magnitude of the graded potential produced reflects the magnitude of the stimulation. Perhaps most importantly, graded potentials are analog, meaning that they are infinitely variable, rather than digital, or all-or-none (as is the action potential).
Graded potentials are not the whole story; the postsynaptic neuron must use the inputs it receives for something. The mechanisms by which a neuron sorts out its various graded potentials and decides whether to generate an action potential is called integration. There are at least forty factors which affect integration, including strength of the signal, time course, type of transmission, spike frequency adaptation, accommodation, and threshold; the two main types are temporal and spatial integration.
Temporal integration takes into account the relative times at which the various graded potentials were generated. The standard measure of this is the time constant, t (the Greek letter tau), which is given by the time which must elapse from the generation of a graded potential until Vm reaches 63% of its final value; this ranges from one to twenty milliseconds in most neurons. t can be calculated by multiplying the particular region's resistance by its capacitance.
If a second graded potential begins before t has elapsed, the two graded potentials will be added, producing a larger, integrated potential. If, however, the second graded potential is generated after t has elapsed, the first signal will not affect the strength of the second one. t does not remain the same throughout the cell; it varies depending on such factors as the diameter of the dendrites.
Spatial summation considers the distance between two simultaneously occurring stimuli. It is measured by the Greek letter lambda (l), which is defined as the distance from the original location of stimulation to where the signal (Vm) has decayed to 37% of its original value. The usual value for l is between 0.1 and 5 millimeters. l is given by the square root or the ratio of rm to ra. Just as with t, a second input will be summed with the first if it is generated within l from the first input. If the two inputs are farther apart than l, they will not be summated. l varies within a neuron, as does t.
not covered in lecture
Action potentials are very rapid changes in the membrane potential which originate at the axon hillock of a neuron and travel down the axon to the terminal bouton, where they cause the release of neurotransmitters into the synaptic cleft. The neurotransmitters induce graded potentials in the postsynaptic cell, which may in turn lead to another action potential. Unlike graded potentials, action potentials are actively, not passively, propagated. The mechanisms of this active propagation are voltage-gated ion channels.
Recall that when the resting potential, due to depolarization by EPSPs, reaches a threshold level, an action potential is created. The threshold of activation varies in different parts of the cell; it is lowest at the axon hillock, where the action potential originates. Special sodium channels in the membrane are sensitive to voltage and open up when the membrane potential reaches threshold levels. Both electrostatic force and osmotic pressure cause sodium ions to rush into the cell; in fact, the forces are so great that the membrane potential reaches +20 to +30 mV at an action potential's peak. The depolarization is not only great in magnitude but also extremely short in duration.
Very soon after the sodium channels have opened, they become deactivated again. Now potassium channels open, allowing potassium ions to follow electrostatic force and osmotic pressure by leaving the cell. The repolarization of the membrane which results is less rapid than the depolarization. The potential actually overshoots its original value, becoming hyperpolarizing, and fluctuates several times before returning to its resting value.
Since an action potential is all-or-none, rather than of variable intensity, further inputs cannot change its magnitude; they can, however, change its rate of firing. The absolute refractory period encompasses all portions of the action potential during which the cell is incapable of producing another action potential, no matter how great the stimulus. This is followed by the relative refractory period, during which the cell will fire again if presented with stimuli of greater magnitude than previously had been required to produce a response.
The action potential travels down the axon to the terminal boutons of the cell. The depolarization of one part of the membrane triggers a like response in adjacent membrane regions. In an unmyelinated axon, the signal is continuously regenerated along the entire length of the axon. This is quite expensive in terms of the energy required to "clean up" in the aftermath of an action potential; it is also somewhat slow. Many axons combat these difficulties by being insulated with a myelin sheath. Ions do not flow through areas covered by myelin, so the action potential cannot be regenerated in these areas. To prevent the complete decay of an action potential, the myelin periodically is interrupted by gaps called Nodes of Ranvier. The signal is be regenerated at these nodes, creating what is called saltatory conduction (from Latin saltus, a leap or jump).